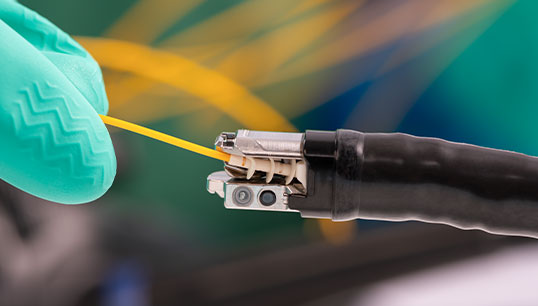
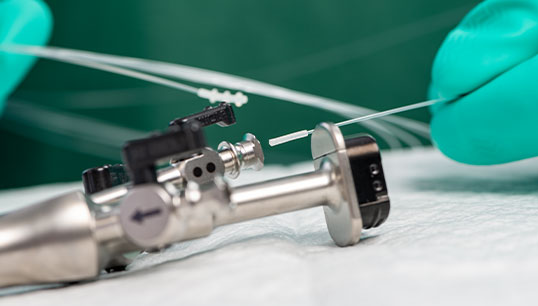
Product Overview
The VERIFY™ RESI-TEST™ SLIDE-THRU Cleaning Process Protein (CPP) Indicator is the first validated qualitative protein detection test for medical device lumens.
Protein is the most common procedural soil found on instruments and is also the most difficult to remove,1 which is why testing for residual protein is essential. Protein can be found in Hemoglobin, Mucous, Body Tissue, Digestive Enzymes, Organs, and more.
If not removed during the cleaning process, residual protein may result in cross-contamination between patients. This testing method can be incorporated into your existing process to help ensure a thorough assessment for rapid and reliable protein detection.
How VERIFY RESI-TEST SLIDE-THRU CPP INDICATOR Works
Quickly confirm if the protein is present in the sample of a medical device lumen to help determine if it can proceed to the next reprocessing phase.
- Detects protein in only a 10-second read time
- Interpret results through a solution color change
- Three brush sizes are available to support a wide range of channel diameters
Why VERIFY RESI-TEST SLIDE-THRU CPP INDICATOR
RELIABLE PROCESS - Take the guesswork out of testing with manual protein detection confirmation that increases assurance and promotes confidence with the testing methods.
- "Protein is the marker most commonly used to evaluate cleaning efficacy"2
- Can be incorporated into your current process
- Color change reference chart aids in visible interpretation of the solution color change
1AJIC American Journal of Infection Control, Oct. 1999, Vol. 41, pp. 245-248. Worst-case soiling levels for patient-used flexible endoscopes before and after cleaning.
2ANSI/AAMI ST79:2017 & 2020 Amendments A1, A2, A3, A4 (Consolidated Text) Comprehensive guide to steam sterilization and sterility assurance in health care facilities.
 United States
United States
 Canada (EN)
Canada (EN) Canada (FR)
Canada (FR) Deutschland
Deutschland Italia
Italia United Kingdom
United Kingdom Australia
Australia New Zealand
New Zealand Singapore
Singapore Brasil
Brasil México
México