Optimizing Sterile Processing Operations
To optimize sterile processing department (SPD) operations, the department needs the resources to be successful and the capability to manage people, processes, and technology effectively. Understanding Organizational Capacity, what resources are needed, and Operational Performance, how to manage the department effectively, are the first steps to improving SPD performance and optimizing operations.
ORGANIZATIONAL CAPACITY
There are seven resources that every SPD needs to be successful. When grouped together, they represent the SPD's organizational capacity:
- Staff
- Technology
- Equipment and Space
- Infrastructure and Utilities
- Instrument Inventory
- Supplies
- Maintenance Programs
When equally balanced and working in harmony, these resources allow the SPD to meet their Customer requirements in the most efficient and clinically compliant manner. When one or more resources are under capacity, under-invested in, or out of balance, the entire process is limited by capacity restraint, and the department struggles to handle the workloads required. Articulating SPD's organizational capacity requirements, the department's current state, and where capacity is insufficient for forecasted workloads is critical to gaining the required approvals to ensure operational success.

Staffing
Planned and unplanned staffing shortages have a significant impact on the SPD's capacity to process instruments, comply with industry standards, follow instrument instructions for use (IFU), maintain staff engagement, and pose a considerable risk to patient safety. Ensuring your department is adequately staffed requires a justifiable and metric-based method to determine full-time equivalent (FTE) requirements that hospital administration will approve. These metrics are key for successfully negotiating staffing budgets that meet operational and clinical requirements.
Steps to Determine Your Department's Staffing Requirements
To determine the department's staffing requirements, follow these six steps:

- Volumes: Calculate the volume of surgical procedures the department will support and the related instrumentation volume for those procedures. Add in other sources of instrument processing workload from clinics, endoscopy, or other departments. For simplicity, volumes are trays, scopes, and peel packs.
- Processing Activities: List the activities the SPD performs and the volumes for each activity per week. Using weekly volumes will take into account variations between mid-week and weekend workloads. This will include all instrument processing activities such as receiving in decontamination, cleaning at the sink, loading washers, inspection and assembly, sterilization, and placing in sterile storage.
- Other Activities: Add in all non-instrument processing activities such as case cart picking, returning unused items from the operating room (OR), meetings, huddles, daily set-up and testing, and any other activity requiring FTEs
- Time Standards: For each activity, determine the reasonable time standard the department can manage and successfully achieve. If time standards are set as goals for staff to aspire to achieve, then build a realistic productivity factor to account for current and acceptable performance.
- Non-Productive Time: Add break times, non-productive or paid time off allowances, and support and leadership positions
- Future Growth: If the department is forecasted to handle growing work volumes in the future, forecast the staffing needs for the future volumes in advance for prior approval
Budget Approvals for SPD Staffing
When asking for budget approval for SPD staffing, especially to increase staff, it's critical to show requirements based on calculated workloads, time standards, and productivity levels that leadership is committed to managing the staff. It's important to convey the clinical and regulatory requirements that the staffing is based on and the impact on patient safety and quality. Lastly, ensure you can articulate the impact of not approving the staffing request.
Learn how STERIS provides SPD consulting services to help facilities calculate their SPD Staffing requirements.
Technology

Technology is the fastest-growing component in many industries, and sterile processing is no different. The SPD's organizational capacity is more dependent on technology than ever before. Its ability to leverage systems to meet capacity requirements while improving operational performance is critical. Instrument tracking systems, such as STERIS's SPM® Workflow Solution and Surgical Asset Tracking Software, are commonly used in many SPDs. However, a typical SPD doesn't utilize its instrument tracking system to its full extent to leverage the system's ability to improve performance and outcomes. To determine your technology organizational capacity, review whether the functionality listed below is available in the software. Discuss whether it is being utilized or considered, as well as SPD's expected use of the functionality.
- Basic Functionality - Includes location scanning and inventory tracking, onscreen assembly count sheets, instrument and tray photos, sterilization load documentation, biological test documentation, recording daily equipment testing, building case carts, and tracking instruments to surgical cases
- Intermediate Functionality - Includes real-time onscreen messaging, cycle selection tracking, quality event tracking, labor productivity tracking, task management, instrument maintenance and repair management, and tracking user competencies
- Advanced Functionality - Includes surgical schedule interface, data analytics and dashboards, guided workflows, AI or visual instrument identification, equipment connectivity, par-level tracking, individual instrument tracking, loaner tray management, static and dynamic IFU instructions
Equipment and Space
Equipment and space are traditionally the main drivers of SPD capacity during the design and construction of a new department. However, they also play a continued role in the organizational capacity long after post-construction. Often undersized, decontamination has come under scrutiny as IFUs require more complex compliance for instrument cleaning and disinfection.
How to Determine Decontamination Capacity
To determine decontamination capacity, you must take the same volumes utilized for staffing purposes and determine the equipment and labor requirements for each step in the cleaning process. It's important to include sink activities such as sorting, rinsing, soaking, flushing and brushing, manual cleaning, and final rinsing. Ultrasonic requirements should also be reviewed for each tray and instrument type to ensure adequate capacity and cycle times are being utilized. Instrument washer capacity is calculated based on washer capacity, cycle times, and workloads requiring automated washing. Lastly, cart washers shouldn't be ignored either to ensure adequate capacity to wash case carts and disinfect instrument containers and utensils.
How to Determine Assembly, Sterilization, and Sterile Storage Capacity
After decontamination, requirements move to assembly, where prep and pack tables, space for instrument staging, and instrument backup inventories are required to maintain a continuous flow. Sterilization space for steam and low temperature is calculated based on sterilizer chamber capacity, cycle times, and workloads requiring sterilization. Sterile storage, case cart staging, and picking are usually included in space requirements. They are impacted by the number of case carts, instrument inventory, and type of storage systems utilized.
To communicate your department’s current equipment capacity, utilizing a line-balancing graph will show each process step's current and required capacity. In the example below, utilized capacity is represented by the blue bars, under capacity in red, and excess capacity in green. This example shows an out-of-balanced SPD where decontamination is under capacity and assembly and sterilization have excess capacity.

Learn more about how STERIS Healthcare Design Services can analyze your department's current capacity, identify project requirements, and create a custom plan to meet the needs and objectives of your department and facility.
Infrastructure and Utilities
Infrastructure and utilities cover the following areas to meet regulatory and infection prevention requirements:
- Heating, ventilation, and air conditioning (HVAC) to meet airflow, temperature, humidity, and air exchange requirements
- Water quality and steam condensate purity
- Electrical needs – including emergency backup power
- Regular environmental cleaning services
- Appropriate floors, walls, and ceilings
Instrument Inventory

SPD organizational capacity is directly impacted by instrument tray inventories, the number of instruments in the trays, and the backup single instrument inventories to complete trays.
- Tray Inventories – Should be sufficient to accommodate surgical schedules without a hardship in processing trays quickly. When the SPD is required to prioritize trays for immediate processing, there may be pressure to deviate from standard processes and additional or different workload requirements that interrupt optimal operations.
- Number of Instruments in the Tray – Directly impacts labor requirements and should be a continuous improvement focus for optimizing trays to remove unnecessary instrumentation. Trays with over 50 instruments usually present opportunities to optimize the tray and reduce the number of redundant and unused instrumentation.
- Backup Single Instrument Inventories – These are required to ensure the SPD can complete the assembly process when an instrument is missing from a tray. Organizing and labeling instrument storage with locations added to the instrument tracking system can help increase staff productivity and organizational capacity.
Supplies
Ensuring the correct supplies are ordered and maintained to meet clinical and regulatory requirements is important. Direct supplies, such as sterilization tape, indicators, personal protective equipment (PPE), brushes, etc., are easy to identify and order. SPD leadership should understand the use of each supply, how it works, the dosage required, usability, and the associated costs. Ensuring the department has adequate supplies to work in a clinically compliant manner is critical. Some supply items for organizational capacity require leadership alignment on the department's standard processes. While supply decisions may not be a regulatory requirement, leaders should standardize their operations and provide the necessary supplies to support the operation.
Maintenance Programs
Equipment uptime and instrumentation that is functional and fit for purpose are essential to maintaining organizational capacity. Every SPD should ensure their equipment and instrumentation is covered under a maintenance program, documentation is maintained for audits, and response times are adequate to repair equipment. There are a few different maintenance programs:

- Equipment OEM Programs – The original equipment manufacturer (OEM) represents the equipment company's recommended maintenance program and optional repair services. Equipment preventative maintenance is designed to prevent equipment failure through regular, proactive servicing. All documentation should be kept and available for audits.
- Equipment AEM Programs – Alternative equipment maintenance (AEM) represents customized maintenance plans based on unique equipment usage. AEMs can be customized to increase maintenance for high-usage equipment and potentially decrease maintenance on low usage. For AEM programs to comply with industry standards and expectations, documentation must be kept justifying the maintenance program.
- Instrument Maintenance Programs – Ensure that the instrumentation utilized by surgeons and healthcare staff is functional and fit for purpose. Preventive maintenance programs covering stainless steel, power devices, and flexible scopes should occur on a pre-set usage interval. Learn more about how STERIS Instrument Management Services supports Flexible Endoscope Repairs, surgical instrument repairs, and surgical device repairs.
Learn more about how STERIS Equipment Service offers a full range of services, including STERIS ConnectCare, which provides connectivity to SPD equipment with computerized documentation systems and on-demand access to maintenance and repair history and real-time equipment performance data.
OPERATIONAL PERFORMANCE
While organizational capacity provides the appropriate budget for staff, equipment, inventory, and other resources, operational performance determines how well the department manages and utilizes people, processes, and technology. Assessing operational performance requires leadership to be open, honest, and transparent when evaluating operations and management systems. The goal is to create a positive culture of continuous improvement with regular audits and assessments.
People
Evaluating a department’s ability to manage and leverage people resources is more than tracking employee productivity. It's an inclusive review of the department's ability to maintain a full staff while building a positive culture based on continuous improvement, compliance, clear expectations, leadership routines, and active education and training programs.

- Technician Staffing – Successful SPDs maintain full staff by creating positive environments that reduce turnover, minimize agency staffing, predict turnover/hiring needs in advance, set clear performance expectations, regularly follow up with staff, and promote knowledge acquisition through certification
- Leadership – Building leadership skills and an effective leadership team is the most critical aspects of improving and sustaining operational performance. Effective leadership starts with ensuring the leadership structure is adequate for the organization's size and needs. Define clear leadership expectations and create specific roles that fit the department's needs. To learn more about the roles and responsibilities of the SPD in building an effective sterile processing program, click here.
- Continuous Improvement – Operational performance cannot succeed without a continuous improvement culture. When evaluating your department, see if performance metrics are tracked and trended with regular audits. Ensure clear and defined improvement goals are set with objectives and root cause analysis. A regular cadence of leadership meetings to review improvement progress promotes leadership accountability and collaboration with the OR and other SPD Customers.
Strong leadership will build a continuous improvement culture. In return, continuous improvement cultures will help build strong leaders.
- Education and Training – Ensuring education and training are strong is paramount to people's operational performance. These programs should be based on standard work so that every employee is taught to perform tasks the same way with clear expectations. Learn more about the importance of SPD education and training programs.
- Culture – The result of people management is creating a positive culture that drives continuous improvement and operational performance excellence. Department objectives and desired cultural attributes should be documented, communicated, and understood by all staff. Department performance, individual objectives, and behavior expectations should be regularly reviewed and clearly communicated. This ensures they understand their impact on overall performance. Both leadership and staff are held accountable for performance and behaviors. Positive cultures do not happen by chance. They are built through purposeful action. Staff engagement is encouraged and occasionally mandated. Leadership follows a formal and regular cadence for employee recognition and team building to create their culture.
Process
The process is split between instruments and operations. Successful performance in both areas is required.
Process – Instruments
Focuses on how instruments are processed from the point of use through sterilization and is all about compliance with standard work. The heart of instrument processing is standardizing how a task should be performed and ensuring compliance with that standard. Manufacturers' IFU clearly define specific tasks that should always be followed, while other tasks are specific to each department and require leadership to document the standard method to perform that task.
Decontamination often has the highest standard work variance due to the numerous instruments IFU requirements and the complexity of cleaning and disinfecting instruments. Standardizing assembly processes focuses on the level of inspection expected to be performed, handling missing/extra instruments, and documenting tray information in the instrument tracking system. When evaluating operational performance - walk through each process step and ask what the standard work is and how compliance is monitored and ensured. This will provide insight into performance and highlight opportunities for improvement. Simply asking staff to follow IFU becomes a monumental task when realizing there are hundreds, some of which are multiple pages long. Utilizing technology workflow systems assists in bringing IFU and standard work information to the technician at their workstation to improve quality and compliance.
Process – Operations
Focuses on the management systems utilized to ensure department processes and operations are performed according to plan. For many SPD leaders, their past experience and knowledge focused on clinical instrument processing with little formal education and training on systems required to run and manage their department's business. Fortunately, operations management is similar to many other process-based industries. Leaders can leverage information on production management, lean methodologies, and other materials to expand their knowledge. Five areas that stand out in process operations are:
- Standard Work and Checklists – These are the foundation of every management system utilized to ensure SPD processes and operations are performing according to plan. If you haven't standardized your work, you can't incrementally improve it, hold staff accountable, and measure outcomes against it. Daily checklists provide a low-cost and easy-to-implement process control to ensure tasks are completed timely. Document all daily decontamination tasks to be performed on a checklist, place the order in which they are to be performed, including standard work on how to perform them, set time and quality expectations, and follow up to ensure staff compliance. These simple steps can help create a culture of compliance that can resonate through the department.
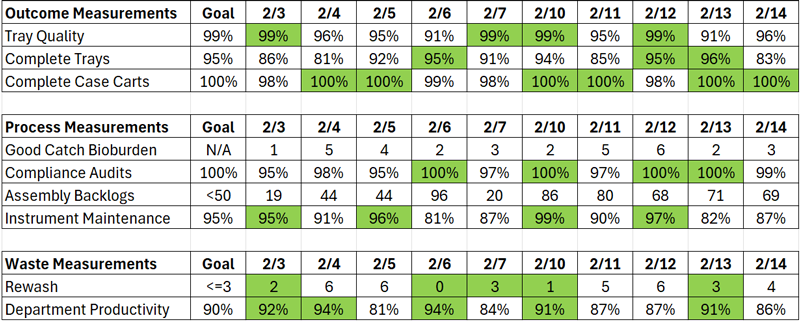
- Performance Metrics – Not every process is measured the same way. There are three performance metrics to consider in improving performance: outcomes, process, and waste measurements.
- Outcomes Measurements – Tell you how satisfied your Customer was and the quality of the products and services provided. They are lagging measurements in that they measure performance that has already occurred. Typical outcome measurements include product quality or tray errors, complete tray or missing instruments, and complete and on-time case carts.
- Process Measurements – Provide information about the product or service before it reaches the Customer; thus, changes can be made to correct the situation and improve the final product. While outcome measurements are required and common, process measurements are perhaps more important to focus on and improve. They help drive continuous improvement within a process and identify specific items to improve. Typical measurements include bioburden caught in assembly, process audits measuring compliance to standard work, backlog of instruments, and instrument maintenance completion.
- Waste Management – Tells leadership how well the department utilizes resources or, conversely, generates waste. These measurements are the most difficult to measure but can provide valuable insight into optimizing operations. The most common measurement is employee productivity, with the understanding that less than 100% highlights opportunities for improvement to eliminate waste. Rework, such as rewashing an instrument due to continued bioburden, is a common waste measurement.
- Daily Production Management – Focuses on prioritizing work that needs to be completed to meet the OR and other SPD requirements. To be successful, there should be consistent collaboration between departments to understand daily instrumentation needs. Leadership should be able to forecast resource needs in advance to meet the OR requirements and align staff schedules to the workload arrival patterns. Requirements should follow a standardized documented process to identify and process priorities. A Customer-focused SPD utilizes OR Liaison positions to proactively ensure the OR receives the instrumentation needed on time while providing real-time Customer service to address issues.
- Vendor Tray Management – These instrument trays pose a unique operations management challenge. SPDs typically do not know in advance the vendor trays required for surgeries. The use of technology can provide significant benefits by offering transparency to surgeon vendor tray orders, status of tray delivery, case date/time, and tracking trays through the reprocessing process to the case. SPM Loaner Solution Technology, such as the SPM Loaner Solution, can simplify and streamline vendor tray management and coordinate communication between departments.
- Instrument Management – This covers tray inventory management, tray maintenance management, missing instruments/backup instruments, and collaboration with the OR on tray optimization. The OR and SPD should meet regularly to review the root cause analysis of missing instruments and review tray optimization opportunities to increase inventory where needed, reuse specific instrumentation, and remove non-used instruments from trays. Instrument management also includes formal programs to perform and track instrument maintenance to meet the surgeon's requirements.
Technology
Using technology systems to provide real-time IFU guidance through guided workflows is critical to providing the information technicians need at the point of work.
Operational performance dives into how well the SPD utilizes technology. As instrument tracking systems are commonly used, this is usually the first place to assess. There are three levels listed in the organizational capacity technology section: basic, intermediate, and advanced functionality. While most SPDs use the basic functionality, there are usually opportunities to improve the utilization of intermediate and advanced functionality. To assess the utilization of the software, create a checklist of the functionality and then review how well it's being utilized. Technology use can expand beyond instrument tracking software to include the utilization of borescopes, cleanliness testing verification, insulation testers, and any other technology advancement in the SPD.
Learn more about STERIS SPM Workflow Solution and how we can help increase software utilization to improve operational performance
Summary
To optimize SPD operations, the department needs the resources to be successful and the capability to effectively manage people, processes, and technology. Both organizational capacity and operational performance provide valuable insight into your SPD's strengths, weaknesses, and gaps. Each attribute discussed above can be broken down into sub-categories. From there, you can evaluate and determine what action needs to be taken to improve.

 United States
United States
 Canada (EN)
Canada (EN) Canada (FR)
Canada (FR) Deutschland
Deutschland Italia
Italia United Kingdom
United Kingdom Australia
Australia New Zealand
New Zealand Singapore
Singapore Brasil
Brasil México
México








